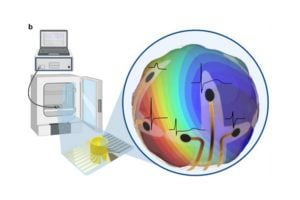
In a step toward understanding how the human heart functions, Johns Hopkins engineers have created a 3D recording device that wraps around lab-grown heart tissues—known as cardiac organoids—to capture their electrical activity. The flexible “shell” microelectrode array (MEA) fits snugly around each organoid, allowing scientists to record how electrical impulses move through the heart tissue in three dimensions, something previous 2D or flat devices could not achieve.
The team’s results appear in Advanced Materials.
“Traditional electrodes only record signals from the surface, missing how those signals travel through the rest of the tissue,” says Department of Chemical and Biomolecular Enginnering PhD student Soo Jin Choi. “Our shell MEA acts like a gentle cage that wraps around the organoid, letting us see how each signal spreads throughout the entire mini heart.”
Each MEA is built using optically transparent material that can fold itself into shape once placed in liquid. The research team designed the MEA with 16 tiny electrodes positioned across the top, sides, and bottom of the shell, ensuring that the whole organoid is monitored. The transparency of the device allows the team to record calcium imaging and electrical activity at the same time.
“This dual capability allows the team to compare electrical signals with visible calcium images and confirm that their recordings accurately reflect the organoid’s beats,” says Choi. “The shell MEA also enables long-term monitoring, allowing us to track electrical patterns for over a week without harming the tissue.”
Long-term monitoring is especially important since the longer cardiac organoids spend in culture, the more they behave like real adult heart tissue. Unlike previous methods, this minimally invasive approach allows for the organoid to stay in its normal growth environment without disturbing it or requiring the use of additional harsh chemicals and dyes.
“The best part of this system is that it gives us a complete 3D picture of how the heart’s electrical signals behave under different conditions. It’s like going from a flat X-ray to a 3D movie of each heartbeat,” says Choi.
The ability to monitor heart activity in 3D opens new possibilities for personalizing platforms for disease modeling, drug testing, and precision medicine. When tested with heart-affecting drugs such as isoproterenol or E-4031, the model detected expected changes in beat rate and signal strength—proving its potential for drug testing and safety studies.
“Our 3D heart-monitoring system opens up entirely new ways to study how electrical signals move through tiny heart tissues,” says David Gracias, professor of chemical and biomolecular engineering and co-author on the paper. “It could help scientists uncover how heart rhythm problems start and bring us closer to personalized treatments for heart disease.”
Looking ahead, the team aims to increase the number of electrodes for even finer resolution. They have also begun to expand the technology to other organ types, such as brain organoids and tissue models. “We’re essentially giving scientists a window into the living dynamics of human tissue that’s going to transform how we understand and treat heart disease,” says Gracias.
Collaborators include Department of Biomedical Engineering associate professor Deok-Ho Kim; alumni Zhaoyu Liu, Engr ’25 (MSE) and Hanwen Wang, Engr ’19 (MSE), ’24 (PhD); and postdoctoral fellow Feiyu Yang; as well as Department of Chemical and Biomolecular Engineering postdoctoral fellow Derosh George.
The work was financially supported by the National Institutes of Health and an MSCRF Post-doctoral Fellowship. There was also support from the National Science Foundation and assistance from the Johns Hopkins Integrated Imaging Center where 3D printed parts of the device were fabricated.