Researchers from Johns Hopkins University and Universidad Carlos III de Madrid have created a computational model that simulates the growth of breast tumors from a biomechanical perspective. Their innovative approach, which factors in properties such as tumor stiffness and density, has the potential to help doctors predict how these cancers might progress and spread. The team’s research appears in Acta Biomaterialia.
The growth of a tumor is not a random process. It is influenced by factors such as the number of cells within the cluster, their size, their access to nutrients, and the surrounding tissue structure. The team’s new model takes these characteristics into account and provides the opportunity to understand how a tumor may evolve.
“This study reveals that the growth and spread of breast cancer is closely tied to the mechanical properties of the tumor and its environment. Understanding these biomechanical forces could eventually inform new therapies that target these forces to thwart the disease,” said research team member Denis Wirtz, Theophilus Halley Smoot Professor in the Whiting School of Engineering’s Department of Chemical and Biomolecular Engineering and vice provost for research.
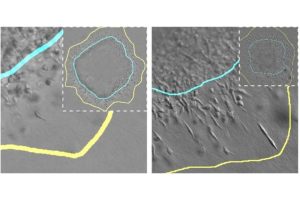
To create their model, researchers began with small balls of cells called spheroids. Spheroids mimic how cells behave in real tumors and can be tested under different conditions, allowing researchers to understand how changes affect cells that make up a tumor.
The team then translated those findings into mathematical equations and input that data into a computer model, enabling them to test in both the computer simulator and the laboratory simultaneously which variables affected tumor growth.
“Our new multi-compartment spheroid system allowed us to control and modulate the system’s biomechanical properties via collagen density and E-cadherin expression, which are known to play a role in breast cancer progression. It was very exciting to work with this team to see the story come together from both experimental and computational perspectives” said Wirtz.
The researchers came away with a few key findings, including that more rigid tumors whose cancerous cells clump tightly together grow larger and spread farther into the surrounding tissue and that when the area around the tumor has dense collagen fibers, it impedes the tumor’s ability to spread.
“While experimentally, proliferation and invasion are often measured as two independent parameters, we observed a strong coupling of these processes. Although they could not be isolated using traditional experimental outputs, the computational model allowed us to study these processes independently and gather insights from the biomechanical properties of our system”, adds team member Ashleigh Crawford, who recently earned her PhD at JHU.
The researchers say that their work holds the potential to improve the quality of treatment and the ability to develop new drugs for breast cancer patients.
“We think that these studies open the door to the development of technologies that allow us to characterize the mechanics of the tumor, which can add relevant information for the choice of cancer therapy,” said Arrate Muñoz-Barrutia, a professor from Universidad Carlos III de Madrid.