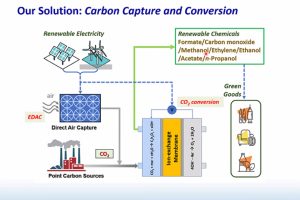
A team led by Chao Wang, associate professor in the Department of Chemical and Biomolecular Engineering and core researcher at the Whiting School of Engineering’s Ralph O’Connor Sustainable Energy Institute, has developed an innovative way to transform carbon dioxide (CO2) into valuable acetate with remarkable efficiency. Hydrocarbons like acetate can be used to manufacture sustainable chemicals and fuels.
“The key is to arrange individual copper atoms on a carbon nitride surface, which results in a carbon-carbon coupling that is more efficient than traditional techniques,” said Wang, whose team’s results appeared in Science Advances.
“Scientists used to believe that only a continuous copper metal surface can do this, but we were curious what happened when you break it down and you only have one copper atom. Can you still induce carbon-carbon coupling?” The answer, the team found, was a resounding “yes.”
This single-atom approach also enables researchers to stabilize and guide specific parts of the chemical reactions more precisely, allowing them to produce acetate while suppressing the formation of other products such as ethylene and ethanol.

The method yielded around 50% useable acetate, which exceeds that of commonly used methods. The team says that these findings could pave the way for more eco-friendly and efficient methods of producing crucial substances, promoting sustainable chemistry and carbon-negative processes.
Wang’s research focuses on carbon capture and conversion to address sustainability challenges associated with climate change.
“Extreme weather situations like wildfires and smoke haze, as consequences of increasing concentration of carbon dioxide in the air, are happening more and more often in recent years,” he said. “It’s urgent for us to develop new technologies to reduce emissions and even remove carbon dioxide from the air.”