A research team that includes members from the Whiting School of Engineering’s Department of Chemical and Biomolecular Engineering has revealed how polymer molecules attached to tiny crystal particles can control how these particles arrange themselves into larger structures called superlattices. By manipulating the size of the nanocrystals, the length and density of polymer grafts, and the rate of solvent evaporation, the team successfully engineered 3D nanostructures with distinct characteristics, offering a blueprint for designing next-generation materials with customizable properties.
The team’s results appear in Science Advances.
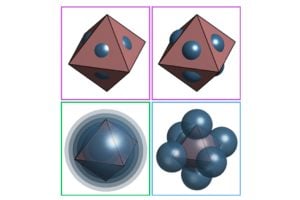
Polymer-grafted nanocrystals are tiny particles coated with long molecules called polymers that can be manipulated into new shapes and materials. In this study, the researchers worked with eight-faced nanocrystals called nanooctahedra. By changing the properties of their polymer coatings, the team made the particles form different structures and patterns, essentially creating materials with customizable properties.
“We found that by tweaking the polymer chains in different ways, such as the weight or lengths, we can control the arrangement of these tiny particles, essentially guiding them to form precise, ordered structures,” said co-author Jarett Ren, a fourth-year student in chemical and biomolecular engineering. “It’s like having a molecular blueprint for constructing advanced materials.”
The team set out to understand how they could manipulate these chains to prompt the nanooctahedra to self-assemble into superlattice structures. They found that changing the length of the polymer chains or how densely they are packed around the nanooctahedra can shift the final material’s structure.
During their research, the team also discovered the importance of the square hexagonal (SH) superlattice phase.
“The SH phase was previously thought to only be a minor phase, but our research found that this structure became stable when polymer chains were tightly packed around particles,” said Ren. “This is a major finding in creating very regular, structured materials—we essentially turned what was considered a useless phase into a controllable building block.”
The study also included computer models to simulate how the polymer chains distribute themselves around the particles. These models helped confirm the team’s findings, showing how small changes in the polymer chains could influence the entire material’s structure.
“By understanding and controlling how these tiny particles self-assemble, we can create materials that are not just functional but are designed from the ground up to perform in exactly the way we want them to,” said team member Thi Vo, assistant professor of chemical and biomolecular engineering. “Understanding these behaviors helps us design new materials with customizable properties, which could be useful in areas such as advanced materials and technology.”
Collaborators include Baixu Zhu, Jun Chen,Yi Wang, Yaxu Zhong, Yang Liu, and Xingchen Ye of Indiana University; Ruipeng Li of Brookhaven National Laboratory; and Akira Yasuhara, Mayu Kakefuda, and Yoshitaka Aoyama of JEOL Ltd.
This work was supported by the US National Science Foundation, US Department of Energy, the Mann Fellowship, the Carroll Family Fellowship, and the College of Arts and Sciences Dissertation Research Fellowship at Indiana University.