Authors: Michael A. Harris, Huihui Kuang, Zachary Schneiderman, Maple L. Shiao, Andrew T. Crane, Matthew R. Chrostek, Alexandru-Flaviu Tăbăran, Thomas Pengo, Kevin Liaw, Beibei Xu, Lucy Lin, Clark C. Chen, M. Gerard O’Sullivan, Rangaramanujam M. Kannan, Walter C. Low, Efrosini Kokkoli
Effective treatment of glioblastoma remains a daunting challenge. One of the major hurdles in the development
of therapeutics is their inability to cross the blood-brain tumor barrier (BBTB). Local delivery is an alternative approach that can still suffer from toxicity in the absence of target selectivity. Here, we show that nanotubes formed
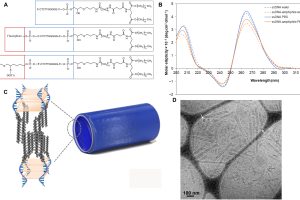
from self-assembly of ssDNA-amphiphiles are stable in serum and nucleases. After bilateral brain injections, nanotubes show preferential retention by tumors compared to normal brain and are taken up by glioblastoma cells
through scavenger receptor binding and macropinocytosis. After intravenous injection, they cross the BBTB and
internalize in glioblastoma cells. In a minimal residual disease model, local delivery of doxorubicin showed signs
of toxicity in the spleen and liver. In contrast, delivery of doxorubicin by the nanotubes resulted in no systemic
toxicity and enhanced mouse survival. Our results demonstrate that ssDNA nanotubes are a promising drug
delivery vehicle to glioblastoma.