Johns Hopkins engineers have discovered that two proteins essential for cell division also help cancer cells metastasize, or spread, especially through tight spaces. The proteins anillin and Ect2, usually found in the cell nucleus where they control the final phases of cell division, also operate in the cell’s cytoplasm—the gel-like compartment encasing the nucleus—to drive the spread of cancer through surrounding tissues and blood vessels.
The team’s results, which appear in Nature Materials, have the potential to help researchers identify new drug targets that could prevent cancer cells from metastasizing.
“We have discovered that anillin and Ect2 are essential for cancer cells to disseminate from their primary site and invade surrounding tissue,” said study leader Avery T. Tran, Engr ’25 (PhD). “When both proteins are disrupted, cancer cells move more slowly, fail to invade, and form smaller tumors.”
Tran worked with Emily O. Wisniewski, Engr’21 (PhD), Panagiotis Mistriotis, Engr’19 (PGF), and senior author Kostas Konstantopoulos, the William H. Schwarz Professor in the Whiting School of Engineering’s Department of Chemical and Biomolecular Engineering and a core researcher at the Institute for NanoBioTechnology, on the research.
Their work demonstrates that anillin and Ect2 work together to activate RhoA, a molecule responsible for controlling how cells move and contract. When these proteins function properly, they enable cancer cells to break through their primary tumor and invade neighboring tissues, a critical step in the metastatic process. However, when either protein is disrupted, as seen in mutated versions of anillin and Ect2, the invasion process is severely limited.
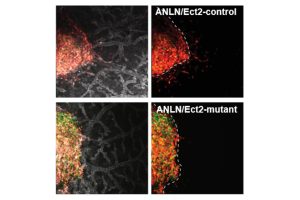
“Through imaging techniques, we tracked the movement of cancer cells in real time in both 2D and 3D-like environments, including a chick embryo model designed to simulate tumor growth and invasion,” said Tran. “We found that cells with higher levels of anillin in the cytoplasm were more efficient at invading surrounding tissue, while those with lower levels of anillin or with mutations in both anillin and Ect2 displayed significantly reduced invasive potential.”
The team also observes that cancer cells with functional anillin and Ect2 display increased levels of these proteins at the cell edges, driving movement and enabling the cells to invade tighter, more confined spaces. This behavior mimics the way cancer cells move through the body during metastasis.
“We are excited to find that when we tested the cancer cells’ ability to escape from blood vessels and spread throughout the body, cells lacking anillin and Ect2 failed to penetrate through the blood vessel walls effectively,” said Tran. She said the finding highlights how targeting this pathway could prevent the spread of tumors.
“We identified a new role for anillin and Ect2 in regulating cancer metastasis,” said Konstantopoulos, “By blocking anillin and Ect2’s ability to regulate RhoA, we might be able to stop cancer cells from invading and spreading to secondary sites, significantly improving patient outcomes.”
This work was supported by the National Institutes of Health, the European Research Council, the Bird Dogs Chair in Translational Oncology at the University of Alberta, the postdoctoral fellowship from the Fonds de recherche du Québec–Nature et technologies, and the Natural Sciences and Engineering Research Council of Canada.
Study co-authors include PhD candidates Alice Amitrano, Bhawana Agarwal, and Sanjiban Nath from the Department of Chemical and Biomolecular Engineering and Institute of NanoBioTechnology and Brent Ifemembi Engr ‘25 (PhD), Se Jong Lee Engr ‘23 (PhD), Kaustav Bera Engr ‘22 (PhD), Yuqi Zhang Engr ‘22 (PhD), Soontorn Tuntithavornwat Engr ‘21 (PhD),; postdoctoral fellows Alexandros Afthinos and Yi Zuo; and Alexander Kiepas, Engr ’22 (PGF), John J. Jamieson, Engr ’21 (PhD), and Daniel Habib, A&S ’21. Co-senior author Petr Kalab and co-author Pei-Hsun Wu are associate research professors in the Department of Chemical and Biomolecular Engineering. Luo Gu is an assistant professor in the Department of Materials Science and Engineering and an associate professor at the Institute for NanoBioTechnology.
Study co-authors also included collaborators at University of Alberta, Radboud University Medical Center, University of Maryland School of Medicine, Duke University, and UT MD Anderson Cancer Center.