The same dye that gives jeans their iconic blue color might also be a game-changer in the fight against climate change, according to researchers at Johns Hopkins Whiting School of Engineering and the Samsung Advanced Institute of Technology’s Air Science Research Center.
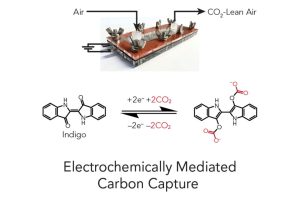
In Advanced Functional Materials, the team introduced a highly modular and scalable solution utilizing electrochemically mediated carbon capture (EMCC), an innovative method that harnesses electricity to activate indigo dye, enabling it to efficiently remove carbon dioxide from the atmosphere.
“Our tests showed that this indigo-based system works at 80% of the best possible efficiency when tested in a simulated environment similar to a factory’s exhaust stream,” said Krish Jayarapu, a co-author of the study and an undergraduate in the Whiting School’s Department of Chemical and Biomolecular Engineering. “The chemical properties of indigo allow the dye to grab onto CO2 when electricity is applied, which means electrons are added to the system. It releases CO2 when the current is reversed, or when electrons are removed. Using electricity for carbon capture, such as in this process, could allow it to integrate as a plug-and-play unit with existing renewable energy sources.”
Through prototype optimizations and simulations, the researchers found that various factors, such as gas flow rate and electrode thickness, influence the efficiency of CO2 capture. However, these factors must be carefully balanced to achieve optimal carbon capture performance for specific operational requirements.
“The device showed promising results for repeatedly capturing and releasing CO2, significantly reducing the need for maintenance-intensive equipment to be involved. This simple and scalable cyclical process is the key to practical carbon capture technology,” said Jayarapu. “Simply put, our indigo-based system has the potential to work economically and under practically relevant conditions.”
Team members say that their indigo-based approach could become an efficient alternative to traditional heat-based methods of CO2 capture, which consume a lot of electricity.
“Indigo dye is cost-effective and readily available, offering a viable alternative to traditional heat-based methods of CO2 capture,” said Yayuan Liu, principal investigator of the study, assistant professor of chemical and biomolecular engineering, and an associate member of the Ralph O’Connor Sustainable Energy Institute. “Free from the limitations of complex equipment, the electrochemically mediated carbon capture method can be utilized in various settings, from small devices to large industrial systems. Moreover, the electrical process helps move us closer to the best possible efficiency for capturing carbon.”
Jayarapu’s research was funded in part by the department’s Elnora Streb Muly Research Award, which supports undergraduate chemical and biomolecular engineering students in tackling practical industry problems.
Other co-authors of the study include Anmol Mathur, Xing Li, Andong Liu, and Lingyu Zhang—all of Johns Hopkins’ Department of Chemical and Biomolecular Engineering— as well as Jaeeun Kim, Hyunah Kim, and Su Keun Kuk of the Air Science Research Center.