Johns Hopkins engineers have discovered how the thickness of fluids surrounding stem cells can affect their behavior and development—insights that have the potential to enhance therapies for bone healing and tissue regeneration. The team’s results, “Extracellular fluid viscosity regulates human mesenchymal stem cell lineage and function,” appear in Science Advances.
“We found that the viscosity – the thickness – of the fluid around stem cells can have a big impact on how they develop, opening up new possibilities for advancing regenerative medicine,” said Alice Amitrano, a doctoral student in the Whiting School of Engineering’s Department of Chemical and Biomolecular Engineering, who led the study with Qinling Yuan, also a doctoral student in the department.
Supported in part by the National Institutes of Health, the team explored how extracellular fluid viscosity affects human mesenchymal stem cells (hMSCs), which are found in tissues such as bone marrow, umbilical cord tissue, and fat, and are uniquely capable of developing into various cell types, including bone and fat cells.
Scientists have long understood that the stiffness of the surface on which these cells are grown plays a major role in guiding what type of cells they become. Soft surfaces tend to lead to fat cell development, while stiff surfaces promote bone cell formation.
Previous studies investigated hMSCs behavior in fluids with a thickness similar to that of water. But in the human body, fluids around tissues have a higher viscosity, with thickness increasing even more at tumor sites.
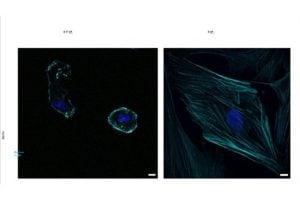
Amitrano and her colleagues took a new approach, studying the cells in fluids of varying thicknesses, including one similar to that of the human body, by adding a substance called methylcellulose to the nutrient-rich liquid that supports cell growth in the lab. They discovered that increasing the viscosity this way caused hMSCs on soft gels—which would typically develop into fat cells—to switch and become bone cells instead.
“We found that increasing the viscosity of the surrounding fluid caused cells that would normally become fat cells on soft surfaces to instead turn into bone cells,” said Amitrano. “These observations revealed that the change in differentiation was triggered by the higher viscosity, which activated signaling pathways and proteins that promote cell development. In other words, viscosity is a key physical property of the surrounding environment; it plays a role that can be just as, if not more, influential than surface stiffness.
The team also found that these changes were not temporary; “the cells retained the effects of the higher viscosity for at least nine days, even after the medium returned to normal, a phenomenon known as ‘mechanomemory,’” said Yuan.
“This ability to control stem cell differentiation in response to viscosity could open new avenues for treating bone-related conditions and tissue regeneration. We hope this research will have a positive impact on stem cell therapies,” said Konstantinos Konstantopoulos, William H. Schwarz Professor in the Department of Chemical and Biomolecular Engineering and a core researcher with the Whiting School’s Institute for NanoBioTechnology..
Study co-authors included Department of Chemical and Biomolecular Engineering PhD students Bhawana Agarwal and Anindya Sen; Department of Biomedical Engineering PhD student Yoseph W. Dance and Assistant Professor Jude M. Phillip; and Department of Materials Science PhD student Yi Zuo and Assistant Professor Luo Gu.
This work was also supported by the Maryland Stem Cell Research Fund and the American Federation of Aging Research.