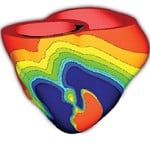
 “We want to be able to see a behavior that’s happening at the level of the whole heart, but also we want to have the ability to zoom in and see the molecular processes and what’s going on in individual cells,” Trayanova says. “I like to describe what we end up with as something like a Google Map—or perhaps a Google Heart.”
“We want to be able to see a behavior that’s happening at the level of the whole heart, but also we want to have the ability to zoom in and see the molecular processes and what’s going on in individual cells,” Trayanova says. “I like to describe what we end up with as something like a Google Map—or perhaps a Google Heart.”
Trayanova dubs the heart “the most developed of the virtual organs” and points to structural qualities that make the heart an organ that lends itself to modeling in ways other organs don’t.
“When you think about the brain, the neurons there are connected at the physical points where they meet,” she says. “But in the heart all cells are really connected together, both electrically and mechanically. If something happens in one portion of the heart, very often the rest of the heart will experience something as a result. It’s like what we in our field call syncytium, meaning it’s acting as if it were a single cell.”
Late last year, Raimond Winslow, the Raj and Neera Singh Professor of Biomedical Engineering and director of the Institute for Computational Medicine, was the lead author of a paper in Science Translational Medicine that described this sort of modeling work as a field ready to emerge into the mainstream of medicine. Models such as Trayanova’s heart, stitched together piece by piece over the course of decades, are now putting computational scientists in position to deliver breakthroughs that matter at the patient’s bedside.
“We want to be able to see a behavior that’s happening at the level of the whole heart, but also we want to have the ability to zoom in and see the molecular processes and what’s going on in individual cells. I like to describe what we end up with as something like a Google Map— or perhaps a Google Heart.” Natalia Trayanova
“We are poised at an exciting moment in medicine,” the paper concluded. “Computational medicine will continue to grow as a discipline because it is providing a new quantitative approach to understanding, detecting, and treating disease at the level of the individual.”
Trayanova seems to have a penchant for making a magnetic first impression.
The first time Patrick Boyle met her, he was a graduate student at the University of Calgary, and he was giving his first-ever major presentation at a conference in France.
“I was so nervous, just shaking all over while giving this talk,” he says. “Plus, I was jet-lagged, and I was wearing a suit that was two sizes too small. After I finished, this woman I’d never seen before makes a beeline for me and shouts ‘You must be BOYLE!’ while giving me this big hug. I remember it like it was yesterday.”
Trayanova had heard good things about Boyle from Edward Vigmond, a former postdoctoral fellow of hers who at that time headed the Calgary lab where Boyle was studying. When it came time to apply for postdoctoral work, Boyle had Hopkins at the top of his wish list. He arrived in the fall of 2011.
“The work she’s doing here is very, very groundbreaking stuff,” Boyle says. “And it’s all moving forward because of Natalia’s amazing perseverance. The sheer will she has had over the years to keep pushing forward with this mammoth undertaking is really amazing.”
From the outset, Trayanova’s lab has been focused on modeling strategies that examine the basic mechanisms of heart disease and shed light on what’s going on and why in an ailing heart. But Trayanova has always had her sights set on the step beyond that as well—delivering clinical innovations. That’s an area where her team is now making exciting progress.
“What we want to do is bring computational tools to the bedside,” Trayanova says. “In order to do that, we developed a way of taking MRI and CT scans of patients and using them to construct a Google Heart that’s specific to each individual.”
How might such a patient-specific model be used? Researchers in Trayanova’s lab are currently working with cardiologist Henry Halperin in the School of Medicine to test whether these models can help physicians make better treatment decisions with patients suffering from ventricular tachycardia.
http://www.youtube.com/watch?feature=player_embedded&v=RzwZ6Af1v1U
Cardiac ablation, the treatment of choice for this potentially life-threatening arrhythmia, aims to destroy or at least scar the tissue in the heart that’s triggering the problem. But finding the right tissue to target is a challenge. Currently, physicians send patients to the electrophysiology lab for extensive testing with an electric probe. Those tests last between four and 12 hours, but they have a success rate of just 58 percent on the first go-round.
Graduate student Hermenegild Arevalo reports that the patient-specific model predictions of optimal ablation targets beat that success rate by a wide margin in retrospective tests in both pigs and humans. Prospective tests in pigs and humans are now under way.
“The ultimate test for this technique will be trying to do prospective tests in humans,” Arevalo says. “We’re gearing up for that right now.”
A second clinical application aims to better identify which patients need defibrillators because of infarctions that put them at risk of developing arrhythmias. Currently that decision is made by calculating an “ejection fraction”—a measure of how much blood the left ventricle of the heart is pumping. If the number comes in below 35 percent, then the patient receives a defibrillator.




