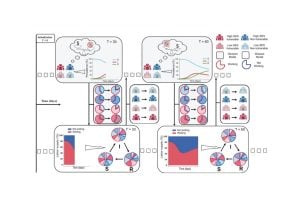
In the past few years, a wave of discoveries has advanced new techniques to use T-cells — a type of white blood cell — in cancer treatment. To be successful, the cells must be primed, or taught, to spot and react to molecular flags that dot the surfaces of cancer cells. The job of educating T-cells this way typically happens in lymph nodes, small, bean-shaped glands found all over the body that house T-cells. But in patients with cancer and immune system disorders, that learning process is faulty, or doesn’t happen.
To address such defects, current T-cell booster therapy requires physicians to remove T-cells from the blood of a patient with cancer and inject the cells back into the patient after either genetically engineering or activating the cells in a laboratory so they recognize cancer-linked molecular flags.
One such treatment, called CAR-T therapy, is costly and available only at specialized centers with laboratories capable of the complicated task of engineering T-cells. In addition, it generally takes about six to eight weeks to culture the T-cells in laboratories and, once reintroduced into the body, the cells don’t last long in the patient’s body, so the effects of the treatment can be short-lived.
The new work, reported April 10 in the journal Advanced Materials, is a bid by Johns Hopkins scientists to find a more efficient way of engineering T-cells. The new research findings put scientists a step closer, they say, to injecting such artificial lymph nodes into people and sparking T-cells to fight disease.
“We believe that a T-cell’s environment is very important. Biology doesn’t occur on plastic dishes; it happens in tissues,” says John Hickey, a PhD candidate in biomedical engineering at Johns Hopkins University and first author of the study report.
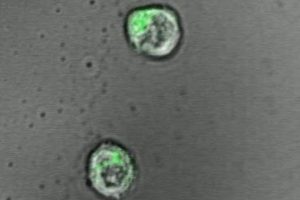
To make the engineered T-cells’ environment more biologically realistic, Hickey — working with his mentors Hai-Quan Mao, associate director of the Johns Hopkins Institute for NanoBioTechnology and Jonathan Schneck, professor of pathology, medicine and oncology at the Johns Hopkins University School of Medicine — tried using a jelly-like polymer, or hydrogel, as a platform for the T-cells. On the hydrogel, the scientists added two types of signals that stimulate and “teach” T-cells to hone in on foreign targets to destroy.
In their experiments, T-cells activated on hydrogels produced 50 percent more molecules called cytokines, a marker of activation, than T-cells kept on plastic culture dishes.
Because hydrogels can be made to order, the Johns Hopkins scientists created and tested a range of hydrogels, from the very soft feel of a single cell to the more rigid quality of a cell-packed lymph node.
“One of the surprising findings was that T-cells prefer a very soft environment, similar to interactions with individual cells, as opposed to a densely packed tissue,” says Schneck.