Johns Hopkins engineers have created a new optical tool that could improve cancer imaging. Their approach, called SPECTRA, uses tiny nanoprobes that light up when they attach to aggressive cancer cells, helping clinicians distinguish between localized cancers and those that are metastatic and have the potential to spread throughout the body.
“Our findings show that SPECTRA—which stands for SERS-based plasmonically coupled nanoprobe for targeted cancer imaging—has huge potential for cancer detection and imaging,” said team leader Ishan Barman, a professor of mechanical engineering at the Whiting School of Engineering. “We’re giving clinicians a more powerful tool that can find cancer cells earlier and more precisely than ever before.”
The team’s research appears in Advanced Functional Materials: “DNA Origami-Engineered Plasmonic Nanoprobes for Targeted Cancer Imaging.”
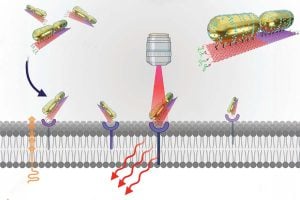
The key to SPECTRA’s success is that it leverages a first-of-its-kind combination: Raman spectroscopy—which uses the scattering of laser light to provide detailed information about molecular vibrations—and DNA origami—which involves folding DNA into specific shapes, as in the Japanese art of paper-folding. The team used the folded DNA as a scaffold to create precisely arranged plasmonic nanoparticles, Raman reporters (a molecule that produces a strong signal when analyzed using Raman spectroscopy), and cancer-targeting DNA aptamer sequences in predefined configurations. These multifunctional nanoprobes were then tested on cancer cells.
The team found that, unlike CT or MRI scans that can indicate the presence of a tumor but not the specific molecular signatures that can alert physicians to current or impending metastasis, SPECTRA effectively and consistently bound to metastatic prostate cancer DU145 cells and even differentiated between those and non-metastatic cells.
In addition, the researchers selected a Raman reporter—a signaling molecule—that results in an active and distinct signal in a range that is usually “cell-silent,” making it stand out clearly against the background of normal tissue, helping clinicians more precisely locate disease.
“It’s a smart design that gives high enhancement to the Raman signal, and it’s uniform,” said team member Swati Tanwar, a postdoctoral fellow in mechanical engineering. “It can distinguish aggressive cancer cells from non-aggressive based on the intensity of the signal. In a tumor, if 10% of the cells are aggressive and 90% are non-aggressive, the 10% will light up and give a very high signal.”
Tanwar said that each strand of DNA in the origami scaffold has a unique sequence and occupies a specific position in the folded origami nanostructure. This meticulous arrangement facilitated the creation of the multifunctional SPECTRA nanoprobe.
“Raman spectroscopy is a molecular fingerprinting tool,” said team member Lintong Wu, a PhD student in mechanical engineering. “Molecules can look similar at a distance, but using Raman spectroscopy they show different peaks and signals throughout the entire spectrum.”
Barman said the team’s design is scalable and more cost-effective than current methods.
“The SERS-based platform is very versatile, and has broad applications,” he said. “It would be a great choice for applications that need a large-scale production of complex nanostructures.”