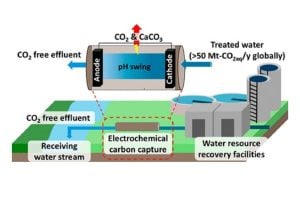
The process of cleaning wastewater—the water that flows down our drains and toilets—can drastically raise carbon dioxide (CO₂) levels in nearby waterways. Two Hopkins scientists have found an innovative way to reduce levels of this common greenhouse gas by running wastewater effluent through a process that uses an electrical current to trigger chemical reactions. This first-ever demonstration of electrochemical CO2 removal from treated wastewater marks a major step forward in decarbonizing water infrastructure. “Exploring the Use of Treated Water in Water Reclamation Facilities for Carbon Dioxide Capture and Sequestration” is published in American Chemical Society ES&T Engineering,
In the United States alone, more than 16,000 plants treat 22.5 billion gallons of sewage daily. The researchers say that widespread adoption of this method could prevent up to 12 million metric tons of CO₂ emissions per year—about 28% of the sector’s total emissions.
“We need to remove greenhouse gases from the atmosphere, and the easiest one to remove is CO2, because it’s most concentrated,” says co-author Ruggero Rossi, assistant professor in Johns Hopkins’ Department of Environmental Health and Engineering. “People say, ‘Let’s build a plant that captures CO2 from the air. Let’s build plants that capture CO2 from the ocean.’ But there’s no infrastructure for that right now. Before you see any of those plants chipping down the CO2 concentration in the atmosphere, it’s going to be a long time, and a lot of money. This is a way to leverage what we already have.”
The Approach: Capturing Carbon with Electricity
To tackle this problem, Rossi and co-author Nakyeong Yun, a graduate student in geography and environmental engineering, developed and tested an electrochemical cell—essentially a device that uses electricity to change the acidity, or pH levels—of water. The cell was placed at the end of the water treatment cycle, at the point where the water was released into the environment. The goal was to capture carbon before it escaped into the atmosphere.
The cell works by creating a pH gradient within the water as it flows through. This chemical shift transforms bicarbonate ions—a form of dissolved carbon naturally present in water—into two capturable forms: CO₂ gas and solid carbonates like calcium carbonate, a stable, chalky compound. Both forms can then be removed and sequestered. The researchers tested this method using wastewater samples from four treatment plants across the U.S., each with different water chemistry compositions.
Advancing the Science: A First-of-its-Kind Strategy
The team members identified key factors such as conductivity and dissolved carbon content that affect performance and optimized the system to work with real-world wastewater.
They also showed that with just a few adjustments—like tweaking the flow rate or electrode spacing—performance could be improved while keeping energy use low. Over 50 hours of continuous operation proved the system was stable, though it did require occasional cleaning to avoid solid buildup inside the cell.
“By fine-tuning the system, we were able to capture more than 57% of the dissolved inorganic carbon—mostly as gas at the anode and partly as solid carbonate at the cathode. We achieved this with energy demands as low as 3.4 kilowatt-hours per kilogram of CO₂, putting our approach on par with or even ahead of many current carbon capture technologies for air or ocean water,” said Rossi.
Staying Carbon-Negative Requires Renewables
Rossi and Yun recognize that because geography, seasons, and even the time of day can change the composition of untreated wastewater, carbon-capture cells are not a one-size-fits-all solution. In addition, since the cells consume energy during operation, they must be powered by renewable energy sources to achieve a net reduction of carbon emissions. Still, the researchers believe that if proven at larger scales, their approach could become a cost-effective and practical addition to global carbon removal strategies, helping cities lower their environmental footprints without overhauling their existing water infrastructure.
“This proof-of-concept study shows the potential of water reclamation facilities in contributing to a better environment, not only cleaning up contaminants from waste streams, but also removing greenhouse gases,” said Rossi.