Hopkins researchers have developed a new method to study how chemical warfare simulants—stand-in chemicals that behave like real weapons without being toxic—break down, offering insights into how better to neutralize real chemical warfare agents (CWAs).
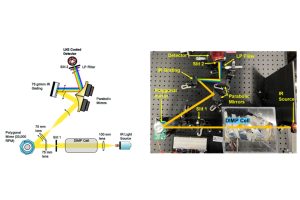
Their Polygonal Rotating Mirror Infrared Spectrometer, or PRiMIRS, imaging system can detect changes in the CWA simulant diisopropyl methyl phosphonate (DIMP) within milliseconds of its exposure to combusting metal powders that produce heat, a potential method of neutralizing chemical weapons.
Their findings, “Development of a dual-spectroscopic system to rapidly measure diisopropyl methyl phosphonate (DIMP) decomposition and temperature in a reactive powder environment,” appear in the Review of Scientific Instruments.
“Neutralizing DIMP with metallic powders happens in less than a second, so we needed to develop a system that could measure very quickly,” said Preetom “Ruku” Borah, a graduate student in the Whiting School of Engineering’s Department of Materials Science and Engineering, who studies the decomposition DIMP after rapid ignition with metal powders. “The spectrometer we developed uses infrared light to capture information more than a thousand times per second.”
Under the guidance of advisor Tim Weihs, professor of materials science and engineering and director of the Materials Science in Extreme Environments University Research Alliance, Borah and colleagues designed the new system to identify the smallest details of DIMP’s combustion, so they could accurately determine the rate and effectiveness of its neutralization.
“With PRiMIRS, we can see molecular signatures change quickly over time as we collect hundreds of measurements every second. We can chart how fast DIMP decomposes with the combustion of aluminum, magnesium, and zirconium composite powders,” says Borah.
The PRiMIRS imaging system uses infrared light to track chemical reactions, shining through a chamber of DIMP vapor interacting with metal powders and reflecting off a series of mirrors to capture rapid changes. A polygonal rotating mirror (which moves at 33,000 rotations per minute), parabolic mirrors, and a grated mirror separate the light into colors that show DIMP decomposition over time. After reflecting off these mirrors, infrared light enters a rotating slit which separates the colors before entering a detection chamber, which correlates each color with exact levels of DIMP in the chamber.
“The polygonal rotating mirror is unique because a typical spectrometer doesn’t change the angle of light onto a stationary grating,” says Borah. “With this feature, we can see wavelengths of light separated into different colors. When combined with the detector, the light variation indicates decomposition down to milliseconds.”
The fully customizable and compact apparatus allows researchers to adjust for different applications, like testing for other CWA simulants.
“Since we have access to change every component of this system, the next best step would be to apply this to different agents. For instance, different simulants may have different molecule structures so we can adjust certain parts to better measure those molecules and still get those rapid results,” Borah says. He posits that the system can also be used for other molecules that require quick measurements but aren’t CWA simulants.
Project collaborators in the Department of Electrical and Computer Engineering included Associate Professor Mark Foster, a fellow at the Hopkins Extreme Materials Institute (HEMI), and postdoctoral fellow Milad Alemohammad. Nick Glumac, a professor at the University of Illinois Urbana-Champaign, along with postdoctoral fellow Austin Butler and graduate student Damon Chen, also collaborated on the project.
The research was supported by the U.S. Department of Defense’s Defense Threat Reduction Agency.