Our cells have a remarkable ability to move through our bloodstream, organs, and tissues—all of which contain fluids. A new Johns Hopkins University study offers important insights into how cells orchestrate these complex movements through the body’s fluid environment: information that has the potential to lead to new medical treatments.
“Cells evolved in water, so they naturally operate according to the principles of fluid mechanics,” said Sean Sun, professor of mechanical engineering at the Whiting School of Engineering and core researcher at the Institute for Nanobiotechnology, who worked with Yizeng Li of Binghampton University on the study. “What our study brought to the table is identifying how pH levels outside the cell can affect the pH level inside the cell, acting like a compass that directs how the cells move and behave.”
The team’s research, “Cell migration dynamics explained by the coupling of mechanics with electrochemistry and pH regulation” appears in Physical Review Research.
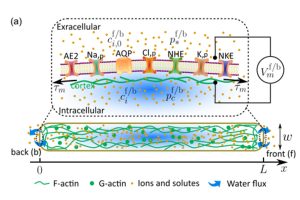
To find out how cells move, Sun created a mathematical model designed to simulate how cells act in various environments, focusing on how charged particles, called ions, move. He and Li then evaluated how different factors, such as varying acidity or pH levels, affected and altered cells’ motion.
They found that the difference in the concentration of chemicals outside the cell uses chemical reactions happening inside the cell fluid (called the cytoplasm) to fuel the movement of the cell membrane. Essentially, an electric exchange takes place, producing momentum in a particular direction, according to Sun.
“The system that’s controlling all the mechanisms involved in generating this movement or force is basically a circuit that’s highly entangled. It’s like a neural network, with inputs and outputs,” Sun said. “What we’re discovering is that cells are taking advantage of the physics of complex mixtures, or the entropy that comes from mixing, and using it to generate force. That is highly unexpected.”
Sun’s model showed that the concentration and behavior of actin—the protein responsible for cell contraction and division, among other things—changes when the intracellular pH is altered. The model also suggests that pH has the potential to indirectly affect cell dynamics through a redistribution of the actin network.
Sun said that his team’s findings might shed new light on how cancer metastasizes.
“Tumors have a very strange pH environment, it’s significantly more acidic,” Sun said. “We’re trying to understand how tumor cells are taking advantage of this in a different way than normal cells.”