The scientific community has long thought that a protein called E-cadherin acts as a “glue” to prevent cancer cells from spreading through the body. However, a new study by Johns Hopkins Engineers reveals that it can actually facilitate breast cancer metastasis.
“We found that E-cadherin itself activates specific metabolic pathways, which spurs the growth of cancer cells,” said study leader Sangmoo Jeong, a core researcher in the Whiting School of Engineering’s Institute for NanoBioTechnology (INBT) and assistant professor of chemical and biomolecular engineering. “These results show a link between E-cadherin and cell metabolism, illustrating that E-cadherin-induced metabolic changes can support tumor growth and spread.”
Their results, “E-Cadherin Induces Serine Synthesis to Support Progression and Metastasis of Breast Cancer”, appear in Cancer Research.
In the past, a hallmark of metastasis was the loss of E-cadherin but recent studies, including “E-cadherin interacts with EGFR resulting in hyper-activation of ERK in multiple models of breast cancer”, done by Denis Wirtz, Theophilus Halley Smoot Professor of Chemical and Biomolecular Engineering and core researcher at INBT, have found that it is not, and some cells still maintain their E-cadherin expression.
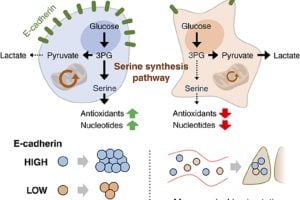
E-cadherin is linked to poorer outcomes in common breast cancers (invasive ductal carcinomas) and pancreatic cancers. It is often detected in patient samples where cancer has spread. Higher levels of E-cadherin promote tumor growth by activating signals between cells and helping cancer spread by reducing harmful oxidative stress.
Jeong’s team found that cells with higher E-cadherin levels are better at coping with damage caused by reactive oxygen molecules, a common challenge for cancer cells to metastasize.
In their study, Jeong and his team used a drug to block the production of serine, a metabolite important for cell growth and reducing oxidative stress, in cancer cells with E-cadherin. This slowed the cells’ growth and ability to spread. By linking E-cadherin to serine production—an important part of cell metabolism—Jeong’s team uncovered the protein’s role in cancer progression.
“Overall, E-cadherin molecules help cancer cells not only grow but also to help manage oxidative stress, which can accelerate their spread,” Jeong said.
He says this finding offers promising targets for inhibiting tumor growth and metastasis in tumors that express E-cadherin. However, Jeong points out that cancer-treatment strategies vary by disease type, and his team’s work focused specifically on E-cadherin’s effect on breast cancers.
“We determined that if we target metabolic pathways, which are dysregulated by E-cadherin expression, then we can prevent or slow metastasis of E-cadherin positive breast cancer cells,” Jeong said.
Study co-authors include INBT core researchers Konstantinos Konstantopoulos and Denis Wirtz, as well as INBT associate researcher Andrew Ewald.