A team including Johns Hopkins materials scientists used computer simulations to demonstrate the link between an alloy’s chemistry and its ability to mitigate radiation damage, a finding that could enable the creation of stronger materials for use in extreme environments, like nuclear reactors or outer space. Their findings were reported in Physical Review Materials.
The team focused their investigation on the atomic structure of grain boundaries, the tiny interfaces where crystals meet in an alloy that can help absorb radiation damage. “Exposure to radiation introduces defects in an alloy that can weaken its performance. Our goal was to investigate if changing the number of elements in an alloy can improve a material’s resilience to radiation,” says Annie Barnett, a PhD student in the Department of Materials Science and Engineering. “We wanted to determine if the alloy’s chemistry influences the behavior of its grain boundaries to limit radiation damage and prevent failure.”
In prior studies, Barnett observed that when grain boundaries are exposed to radiation, they undergo atomic reorganization into denser structures, potentially due to the absorption of defects which extends the lifetime of materials during exposure to radiation. She thought that a material comprising more elements might increase its number of stable grain boundary structures, making it better suited to accommodate defects.
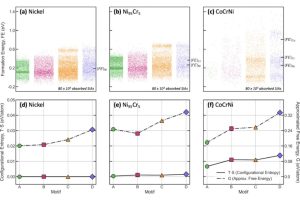
To understand how the materials’ chemistry activated the grain boundaries, the team chose to simulate irradiated alloys of nickel, chromium, and cobalt because when exposed to radiation, the mechanical performance of these materials is superior to that of the steel used in many of today’s nuclear reactors. “Past experiments have shown that this composition maintains exceptional strength and stability under radiation so, given our baseline understanding of its deformation processes, it was an ideal material to model,” says Barnett.
Using pure nickel, a dilute alloy consisting of 95% nickel and 5% chromium, and a complex alloy containing equal parts nickel, chromium, and cobalt, the team ran three molecular dynamics simulations to observe how grain boundary atoms behaved when they were exposed to radiation and therefore developed defects. “By running three otherwise identical models that differed only in their composition, we isolated the role of chemistry in driving grain boundary structural evolution during defect absorption,” says Barnett.
The simulation was conducted on a nanometer scale, providing the researchers with the atoms’ precise locations while the rearrangement occurred within the grain boundary region.
“We learned that grain boundaries in chemically complex alloys mitigate radiation damage more effectively than in the pure or dilute alloys we evaluated because of the greater number of possible atomic arrangements they provide,” says Barnett.
Researchers also found the relationship between defect absorption and alloy chemistry wasn’t linear: there are other variables that affected the alloy’s interaction with radiation damage.
“While we believed chemical complexity would enable grain boundaries that are better defect absorbers, we discovered there are factors, like stress, that also can influence defect absorption,” says Barnett, who now wants to design a lab experiment to better understand boundary structure transitions and which factors cause defect absorption.
This work was led by Michael Falk, professor of materials science and engineering, and the team was led by Mitra Taheri, professor of materials science and engineering and director of the Materials Characterization and Processing (MCP) facility. The team collaborated with Jamie Marian, professor at the University of California, Los Angeles. This research was funded by the United States Department of Energy, Office of Basic Energy Sciences, through the Mechanical Behavior and Radiation Effects program.