Released from their pill’s casing as it dissolves in stomach acid, dozens of star-shaped mini-machines, each the size of a dust mote, sweep into the patient’s small intestine. As these devices pass deeper into the jejunum, body heat starts to melt the paraffin wax holding their six arms in place. Like a starfish gripping its prey, the arms snap shut, latching onto the intestinal wall with a vise-like grip. A spring-like mechanism in the machine, constructed of metal and flexible film, now engages, puncturing the tissue and painlessly injecting a drug dose directly into the patient’s bloodstream.

This revolutionary method of drug delivery would avoid the need to inject medication subcutaneously—a standard route for administering drugs that has come to encompass treatment for a wide variety of illnesses, including insulin shots for people with diabetes, immunomodulators for autoimmune conditions, chemotherapy drugs for cancer treatment, and GLP-1 agonists for weight loss.
“We would have a way to deliver medications without pain,” says David Gracias. “Keeping up on your medications and trips to the doctor would become a lot less stressful.” The idea of these so-called “theragrippers,” or therapeutic grippers, is but one of many to have sprung from the mind of Gracias, a professor of chemical and biomolecular engineering at the Whiting School of Engineering, with a joint appointment in the Department of Oncology at Johns Hopkins Medicine, among other titles.
Gracias is a prolific inventor specializing in miniaturized systems, self-folding materials, and physically intelligent machines that can adapt to dynamic environments, drawing immense inspiration from the living world.
“I think nature is very intelligent. It’s the best engineer,” says Gracias, who is also an associate researcher at the Institute for NanoBioTechnology.

Over the past 21 years at Johns Hopkins, Gracias has made a name for himself in a richly interdisciplinary space where engineered and living systems meet. The breadth of this research is reflected by his elections as a fellow to international societies running the disciplinary gamut from physics to chemistry, medical and biological engineering, and electrical engineering. Late last year he was named a Fellow of the National Academy of Inventors.
Much of the work in Gracias’ lab focuses on developing engineered living materials that could deliver tremendous advances in health care. Toward this goal, Gracias and colleagues often rely on biofabrication, which weds abiological craftsmanship, such as microchip manufacturing, to biological media such as cells.
Numerous examples of wild-sounding, dynamic devices have been born of the Gracias Lab, including:
- miniaturized biomedical robots, actively powered by microsprings wrought of thin films and “hydrogels” that absorb liquid and swell
- origami-like, self-folding hydrogels that could help form artificial, transplantable tissues
- hyper-miniaturized sensors, nicknamed “cell tattoos,” that could adhere to individual cells and report on their health as proxies for larger organ systems
“I like to work on problems that will be important in the future rather than immediate problems which I feel can be tackled by industry,” says Gracias.
Entering the Body with Small Things
“One of the big ideas in medicine—and this has been around in movies—is, ‘Can we enter the body with small things?'” says Gracias. “Clearly if we could, it’s a game-changer.”
One of Gracias’ closest collaborators on the medical side at Johns Hopkins is Florin Selaru, a professor in medicine, oncology, and biomedical engineering. Together, Gracias and Selaru are developing theragrippers to perform a variety of tasks. Their concept owes as much to how macroscopic organisms take up residence in our bodies as to flicks like 1987’s Innerspace, where a miniaturized submersible, piloted by a shrunken Dennis Quaid, maneuvers through Martin Short’s innards.
Theragrippers take a cue from the way hookworm parasites attach themselves to intestinal walls. The grippers can be outfitted with chemical sensors to autonomously shapeshift in the presence of certain sugars, amino acids, DNA sequences, enzymes, pH levels, and more to specifically target certain areas of the body.
These theragrippers could also be used for sustained drug delivery. Even with the advent of extended-release drug formulations, pharmacologists still have been limited in how effective they can make oral medications because of the unavoidably propulsive nature of the gastrointestinal tract, where wavelike muscle contractions known as peristalsis constantly move contents along.
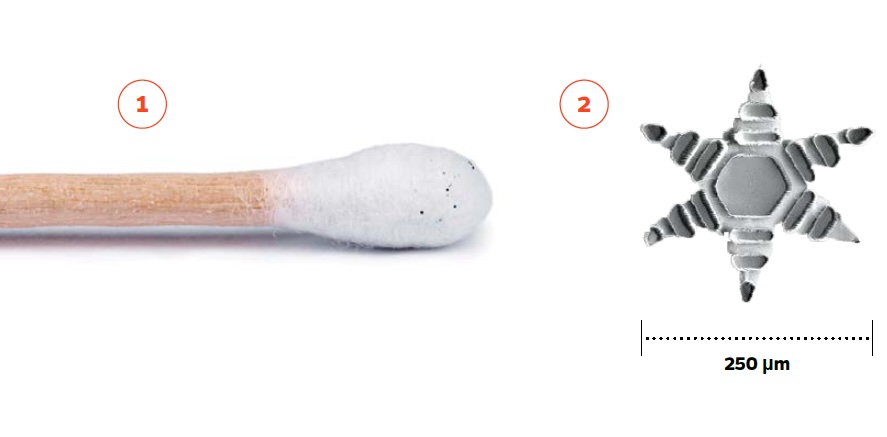
2. When exposed to internal body temperatures, the star-shaped device folds and affixes to the intestinal wall.
“The GI tract is akin to an assembly line; it keeps going on and on,” says Selaru, who is also director of the Meyerhoff Inflammatory Bowel Disease Center. As a result, patients often do not get full doses of even extended-release drugs. “If you wanted to develop a truly sustained release formulation,” says Selaru, “then you would have to essentially latch the ingredient on the inside of the GI tract.” A pill that turns loose a fleet of theragrippers with pharmacological payloads onboard could do just that.
Gracias and Selaru are conducting preclinical testing of theragrippers fashioned in their labs. In one animal model, mice that received a pain-killing drug via theragrippers had the drug in their bloodstreams for 12 hours, versus just two hours for mice given the drug via the normal oral route, as reported in the journal Science Advances.
The little grippers could also be used to conduct canvassing-like biopsies, grabbing tissue samples by the hundreds as they move through the body. In this way, theragrippers could enable robust statistical sampling in a large organ such as the colon, or in hard-to-reach conduits in the body, such as bile ducts, to offer sensitive new disease screening.
By catching cancers and precancerous cell changes early in disease development or progression, clinicians could therapeutically nip the problem in the bud. This strategy potentially could prove more effective at catching malignancies than today’s taking of a limited number of chunky tissue specimens, a comparatively crude approach that can readily miss a small and still-easily-treatable bad spot. Other diseases, ranging from infections to metabolic disorders, could likewise be detected during early asymptomatic stages.
“Florin and I have been working on [the] idea of deploying theragrippers and giving an organ a ‘score’ to get an overall assessment of its health,” says Gracias. “You could potentially find cancers or other diseases much earlier this way.”
Vessel Verisimilitude
Another pioneering project from Gracias is the biomimetic artery—a complexly layered and cellularly tiled tube that functions remarkably like real-life vasculature.
Physiologically, blood vessels are wonders of natural engineering. Although presenting as mere tubes, they are actually composed of three concentric layers of cells, all precisely working in concert. Flat endothelial cells line the vessel’s interior, backed by smooth muscle cells that contract and relax to enable blood flow, all wrapped in fibrous connective tissue cells. “Blood vessels are very complicated,” says Gracias. “They’re not just haphazard.”
Astoundingly, our bodies contain about 100,000 kilometers (60,000 miles) of blood vessels—enough to wrap around Earth nearly two-and-a-half times. This vast network begins as branches of main arteries and veins, narrowing down into arterioles and venules, then wending further down into copious capillaries. Of the 100 trillion-some cells in our bodies, most are no more than a tenth of a millimeter (four-thousandth of an inch) away from a capillary.

A host of major ailments directly or significantly involve blood vessels, including of course cardiovascular disease, but also hypertension (high blood pressure) and cancer. Working with Lewis Romer, a professor of anesthesiology and critical care medicine at Johns Hopkins Children’s Center, Gracias wants to dramatically improve our state of knowledge about vasculature for new insights into disease etiology and treatment.
“We’re interested in blood vessels because blood vessels are everywhere,” says Romer, who is also a professor of cell biology, biomedical engineering, and pediatrics. “The ways in which the walls of those blood vessels are put together have certain themes and certain geometries, and we’ve been trying to recapitulate those with very high-level biomimicry.”
To this end, the two researchers’ labs have invented and patented their biomimetic artery and are continuing to hone its abilities.
Scientists have long struggled to engineer such convoluted devices. Gracias uniquely approached the challenge, leaning in part on his background before Johns Hopkins working in the semiconductor industry. As a senior engineer at Intel, Gracias learned how to fabricate novel circuits, and he invented ways for microchips to operate faster.
He brought that experience to his Hopkins lab, where tools and techniques of the microchip trade, such as photolithography—patterning materials using light—rub elbows with 3D printing and advanced imaging technologies. For the biomimetic artery, Gracias, Romer, colleagues, and students have employed such methods to create self-rolling layers of cells and tubular sensors. The upshot is a circular tube with accurately layered and aligned cells.
The researchers are now putting these biomimetic vessels through their paces by flowing biochemical gels into them containing medicines such as nitrates, which widen blood vessels, and hormones, gauging how the cellular structures respond. “We can better understand dysfunction in the tube and how it occurs by studying these systems,” says Gracias.
This approach offers significant advantages, both practical and ethical, over research in animal models or traditional laboratory setups.
“We’re able to stay true to many of the genetic and biochemical and anatomic parameters that exist in a person that are very different than they are in an animal or in vitro modeling,” says Romer, adding: “This research is a great credit to David’s ingenuity and ability to translate abstract ideas into very basic kinds of design parameters for systems production and engineering.”
‘Science is Magic That Works’
Gracias’ and Romer’s blood vessel mimics could eventually find their way into fully realized, artificially grown masses of cells that work together to function like a bona fide organ. These so-called organoids—another interest area for Gracias—could allow researchers to deeply investigate disease pathologies and drug pharmacokinetics.
“Any great technology, if you saw it 20 years ago, it would’ve seemed like science fiction, right?”
— David Gracias
As with his various micro-medical bots, the idea of living pseudo-organs might sound far-fetched, but Gracias points out that today’s realities were yesterday’s big dreams.
“Any great technology, if you saw it 20 years ago, it would’ve seemed like science fiction, right?” says Gracias. “If you use that same metric and think toward the future, then I think definitely in the next 20 to 30 years, we will go into the body with robots and tiny sensors and it will become very mainstream.”
Through his lab’s boldly innovative and inventive purview, Gracias is continuing to train and inspire young researchers. He recalls how important mentorship was to his own maturation. “It’s a very rewarding part of the job to mentor the next generation,” says Gracias. “That’s something very important about life—you have to have good mentors.”
For Gracias, a critical mentor is George Whitesides, a professor of chemistry and accomplished inventor at Harvard University, where Gracias served as a postdoctoral researcher. “For the first time, I saw that you can actually use your knowledge to create things, which is very empowering,” Gracias recalls. “They often say, ‘Science is magic that works,’ and I always tell students that once you learn the basics, you can use that knowledge to build all kinds of things.”
One student of Gracias’ who took these words to heart is Anum Glasgow, who graduated in December 2009 as a biomedical engineering major. Now an assistant professor of biochemistry and molecular biophysics at Columbia University Medical Center, her research centers on the ubiquitous and quintessential biological self-folding systems of proteins. Often called the “workhorses of biology,” proteins enable a staggering amount of life’s functions and forms. And proteins do all this thanks to the complex shapes they assume after being initially spit out in cells’ ribosomes as a string of amino acids.
Glasgow recalls her undergraduate work in the Gracias Lab in such matters. “What always fascinated me the most about our projects was this self-folding principle,” she says. “My training there was really foundational to what I do now.”
In mentoring students of her own, she is advancing understanding of the principles for protein structure, working toward a goal of rationally designing artificial proteins with desired properties for a slew of applications in medicine and industry. As Gracias did with her and her fellow students, Glasgow is encouraging fresh, innovative approaches.
“The most amazing thing about being an undergraduate researcher in the Gracias Lab is the level of independence that you have in your research,” she says. Glasgow thinks that inventive, independent streak stems from Gracias himself. “He really marches to the beat of his own drum,” Glasgow says.
Indeed, in his research endeavors, Gracias says he continues to find inspiration from solutions honed by the living world, a passion that goes all the way back to his childhood days growing up in India. He remembers marveling over bottled specimens of insects that he and his brother kept preserved in formalin. Even today, he finds himself routinely amazed by trees’ quotidian architectures.
“My son makes fun of me,” says Gracias, “but whenever I’m parked at a traffic light or walking the dog, I admire the trees around us that are often not noticed.” Instilling appreciation for nature’s engineering—honed over millions of years of evolution—is part of Gracias’ vision for the engineers of tomorrow.
“There are all kinds of great ideas, right there outside,” says Gracias. “We humans just have to stop and look.”


