Cardio-Vascular Flows
Computational Modeling of Cardiovascular Flows
Cardiovascular disease (CVD) remains the leading cause of death in the US and future trends are troubling: heart disease has a strong correlation with age and by 2050, more than 40% of the population will be ≥45 years old. Heart disease also has a positive correlation with obesity and since 1980, the percentage of adults aged 20-74 who are considered clinically obese, has doubled to more than 30%. CVD also ranks highest in terms of national healthcare expense with close to half a trillion dollars spent on this in 2010; this is more than twice the health expenditures on cancer, which is the next most expensive disease. Improvements in treatment outcome are not keeping pace with demographic and cost trends and the only way to tackle this burgeoning crisis is to develop new treatment protocols that are not only more effective but actually reduces the cost of medical treatment.
One way to tip the scales in our favor is to counteract these unfavorable trends with other trends that are favorable, and one trend that is significantly in our favor is the Moore’s Law which refers to the trend in computing that is continuously enabling ever-larger, faster and cheaper computations. In our group we are working at the intersection of computation and cardiology to develop tools and insights that can lead to novel simulation-based tools for management of CVD.
Current Projects
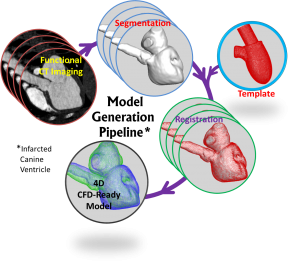
1. Image-Based Computational Models of Cardiac Flows
Recent advances in cardiovascular imaging (cardiac CT, 4D echocardiography and cardiac MR,) open up the possibility of image-based, patient-specific computational models for diagnosis, therapy and surgical intervention. However, significant challenges have to be overcome in order to reach a point where such tools can be brought into the clinical workflow loop. These include registration and segmentation of 4D imaging data, accurate simulation of flow with complex moving bodies and fluid-structure interaction, and validation of computed solutions against clinical data. Our research group is tackling all of these issues.
Collaborators: Laurent Younes (Appl. Math, JHU), Natalia Trayanova (BME, JHU), Theodore Abraham (JHU, Cardiology), Richard George (Cardiology, JHU), Albert Lardo (BME, JHU), Howie Huang (Computer Eng., George Washington U.)
2. Flow Mediated Thrombogenesis in Infarcted Ventricles
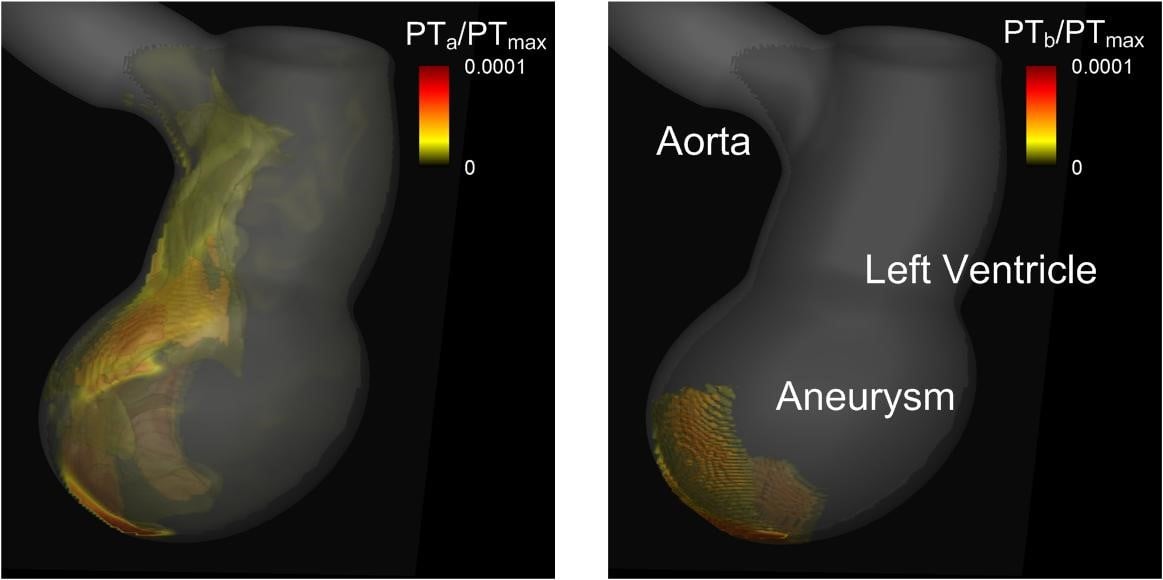
One of the most feared complications for acute myocardial infarction (AMI) is the occurrence of a thromboembolic event due to left ventricle thrombus (LVT) formation. The identification of high risk patients and the pharmacologic prevention of LVT formation are the keys to preventing those embolic events. The development of LVT is promoted by the combination of altered blood flow, endothelial injury, and hypercoagulability, and the generation of thrombus results from a coagulation cascade that is mediated by the dynamics of ventricular flow. We apply a multi-physics computational model to study this flow mediated thrombogenesis in infarcted left ventricles. Blood flow in the left ventricle is simulated by solving the incompressible Navier-Stokes equations and this is coupled to a convection-diffusion-reaction equations based model of platelet activation and coagulation. The thrombus formation patterns are then correlated with the hemodynamic metrics such as wall shear stress and residence time to explore the biophysics underlying LVT risk and its prediction in infarcted ventricles.
Collaborators: Richard George (Cardiology, JHU)
|
Figure: 3D isosurfaces of normalized platelet number density showing distributions of activated (PTa, left) and bound (PTb, right) platelets in a model of an infracted left ventricle with an apical aneurysm. |
3. Effect of Mitral Valve Dynamics on the Intraventricular Flow Pattern
It has been known that the mitral valve configuration could totally change the LV flow patterns, and thus the proper modeling of mitral valve leaflet(MVL) is essential to the accurate simulation of LV hemodynamics. From our computational results, we have found that the MVL dynamics affects to the mitral jet velocity wave propagation, asymmetric vortical flow pattern, and blood mixing and washout. We are performing computational studies of the fluid structure interaction(FSI) of MVL in a realistic LV flow model. The MVL geometrical model is generated from the physiological morphology and considered as a nonlinear hyperelastic membrane with zero thickness and numerically modeled with a subdivision-surface finite element method. The effects of major embedded fibers inside the mitral valve are modeled using inextensible nonlinear fiber with small bending stiffness, while the effects of secondary fibers are considered in the material model used for the valve membrane.
4. Simulation and Analysis of Heart Sounds
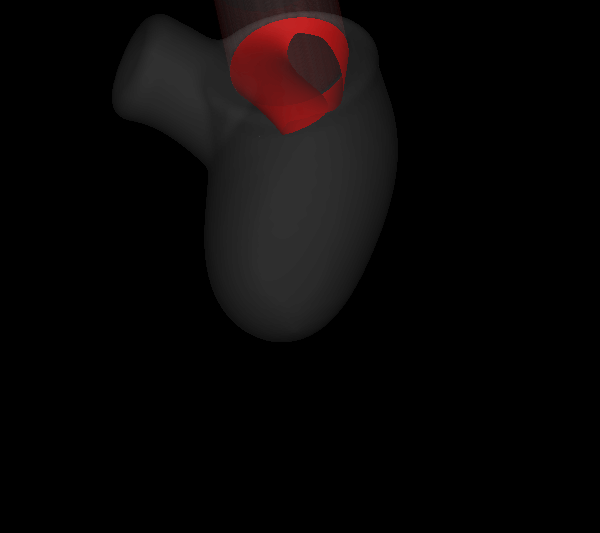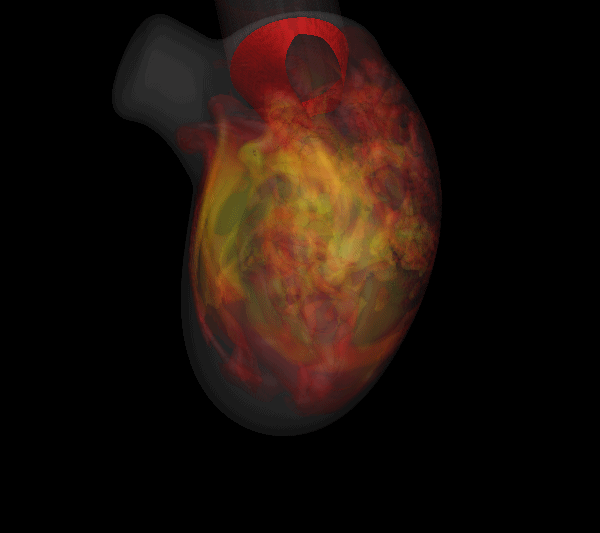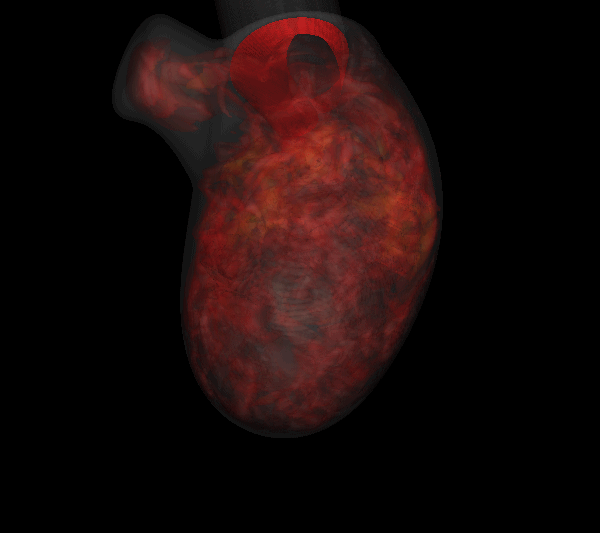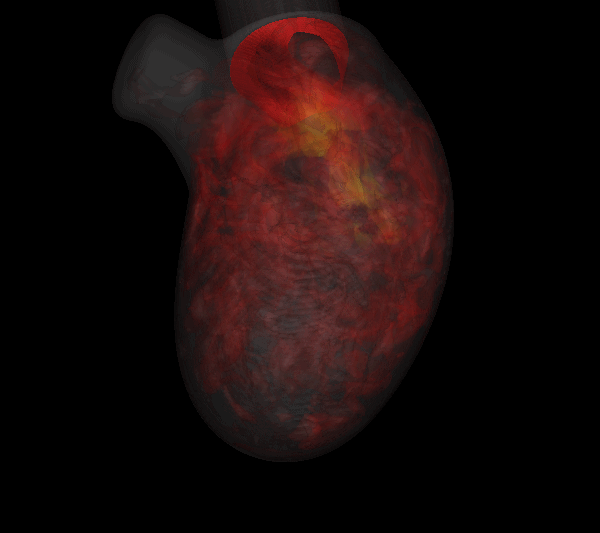
Virtually every heart condition has a distinct acoustic signature which can be traced to abnormal hemodynamics localized somewhere in the cardiovascular system. While the idea of cardiac diagnosis based on heart sounds (known as cardiac auscultation) is not new, no systematic effort has been undertaken to map this. Heart sounds from an individual contain vast amounts of disease related information, but cardiac auscultation currently uses only a small fraction of this information for diagnosis and decision support. Furthermore, cardiac auscultation is a skill in decline newer generations of cardiologist and physicians are not being trained properly in the art and science of cardiac auscultation; a valuable diagnostic modality is therefore falling into disuse and is in need of new insights and paradigms. The use of new ultra-sensitive, low-power, low-cost compact acoustic sensors and signal analysis algorithms, coupled with multiphysics computational modeling and big-data graphical model techniques for pattern recognition, should produce the paradigm-shift needed to bring the centuries old technique of cardiac auscultation back to the forefront of healthcare. We are developing a unique computational modeling approach that takes 4D echocardiographic and magnetic resonance imaging data as input, and simulates cardiac blood flow as well as the associated heart sounds. This will enable us to unambiguously relate heart sounds (measured on the precordium) to the underlying hemodynamic abnormality; a crucial capability that has been lacking in all past efforts in automated cardiac auscultation.
Collaborators: Andreas Andreou (ECE, JHU), William Reid Thompson (Cardiology, JHU), Theodore Abraham (Cardiology, JHU)
 |
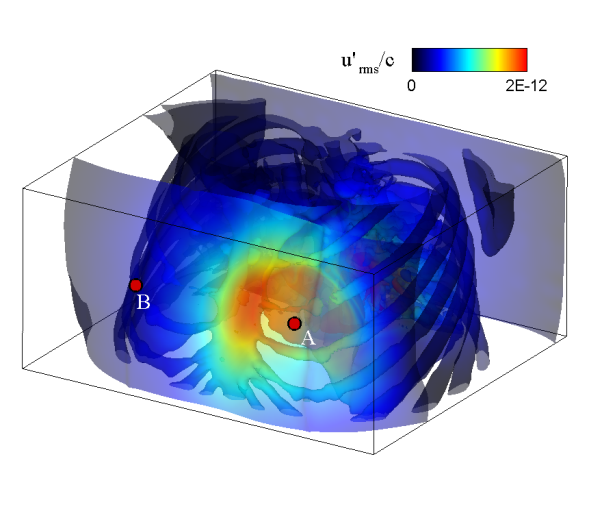 |
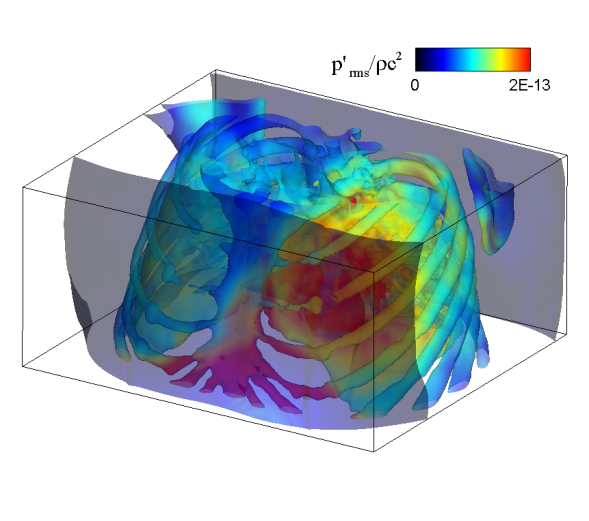
| Simulated results for the propagation of systolic murmur. Root-mean-squared acoustic pressure (left) and velocity fluctuations (right). | |