People
| Graduate Student | Zeng Zhang |
| Project Supervisor | Prof. Joseph Katz |
| Project Collaborator | Prof. Misun Hwang (Children’s Hospital of Philadelphia) |
Introduction
Hydrocephalus is a condition involving abnormal cerebrospinal fluid (CSF) accumulation in the brain ventricles. It is the leading cause of brain surgery in newborns and could result in long-term neurologic disabilities in up to 78% of the cases. Intracranial hypertension is one of the major complications of hydrocephalus, which could lead to compromised cerebral blood flow (CBF), and brain ischemia. The clinical decision for surgical intervention relies primarily on brain imaging using ultrasound, CT or MRI depicting ventriculomegaly along with other clinical symptoms. However, radiographic diagnosis can sometimes be misleading since some of the hydrocephalus patients with elevated intracranial pressure (ICP) do not have a ventricular enlargement. Delayed intervention could lead to cerebral ischemia and permanent brain damage. Moreover, the golden standard of ICP measurement by inserting invasive pressure sensors is rarely used due to the associated infection, catheter tract hemorrhage, and neurological deficits. Hence, there is a dire need for non-invasive biomarkers of elevated ICP and brain ischemia that could guide timely treatment.
In this project, clinical Contrast-Enhanced Ultrasound (CEUS) images are post-processed using a novel PTV method to quantify the relationship between ICP and the spatial-temporal distributions of blood flow in the cerebral blood vessels of pig models. CEUS imaging utilizes intravascular microbubbles (<5μm) with high echogenicity to visualize blood flows in echograms, and PTV is applied to track the bubbles. The bubble trajectories are used to reconstruct the blood vascular maps and measure the blood flow velocities.
Results
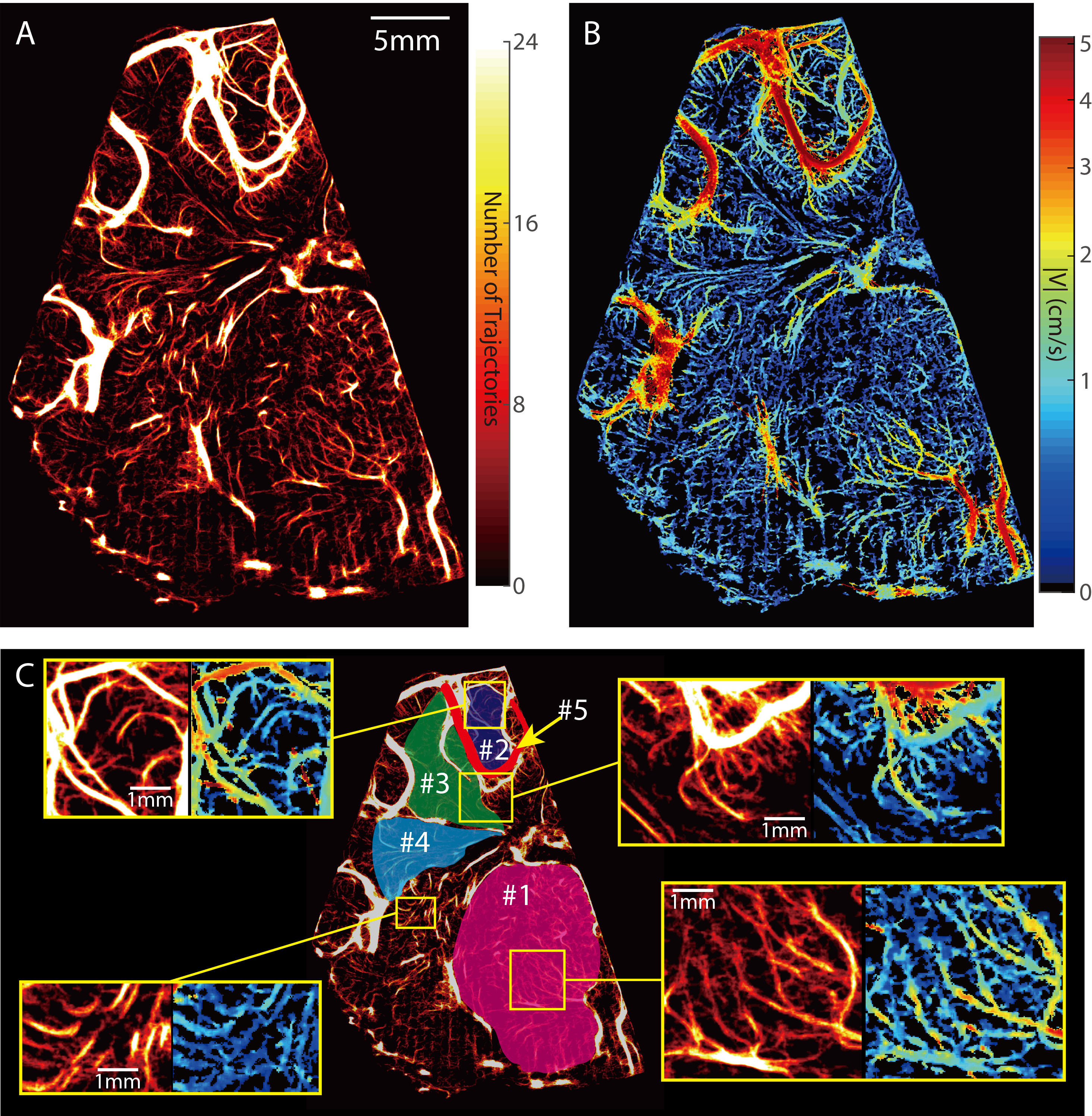
Figure. 1 Sample result for a healthy pig. A. Cerebral vasculature map. B. Cerebral microcirculation distribution. C. A closer look at different regions.
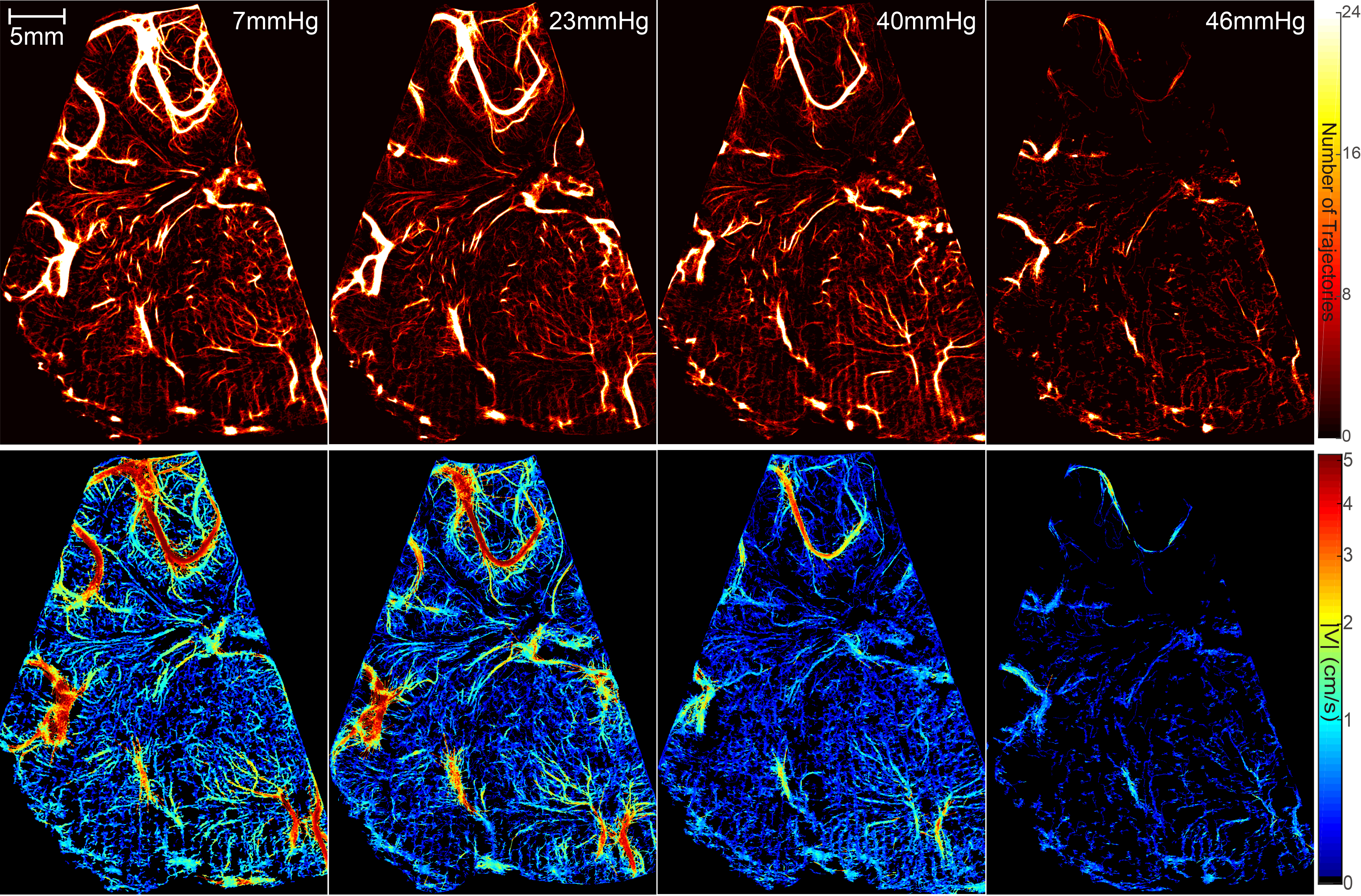
Figure.2 Comparison of cerebral vasculature maps (top row) and velocity distributions (bottom row) for different ICP levels.
In Figs. 1 and 2, the vasculature maps are generated by plotting the heat map of all trajectories that last for more than 4 frames. It is demonstrated that vessels that are separated by ~100μm can be clearly distinguished. Fig. 2 shows that the global perfusion in this view plane gradually decreases as ICP increases. When ICP=46mmHg, the extremely low perfusion could lead to cerebral ischemia.
We have adopted two parameters as surrogates for the flow rate: (i) For characterizing the average perfusion in the micro-vessels in regions 1-4, we introduce the CMC parameter, defined as the time- and spatially-averaged blood velocity (in mm/s) over the entire region, including spaces located within and outside of the micro-vessels. Hence, this parameter accounts for both the density of the vessels as well as the velocity in them. (ii) For the flow in the major blood vessel 5, we utilize the VTI, namely the time integral of spatially-averaged velocity in this vessel, expressed in cm/min. This parameter is analogue to the VTI used in Doppler Ultrasound.
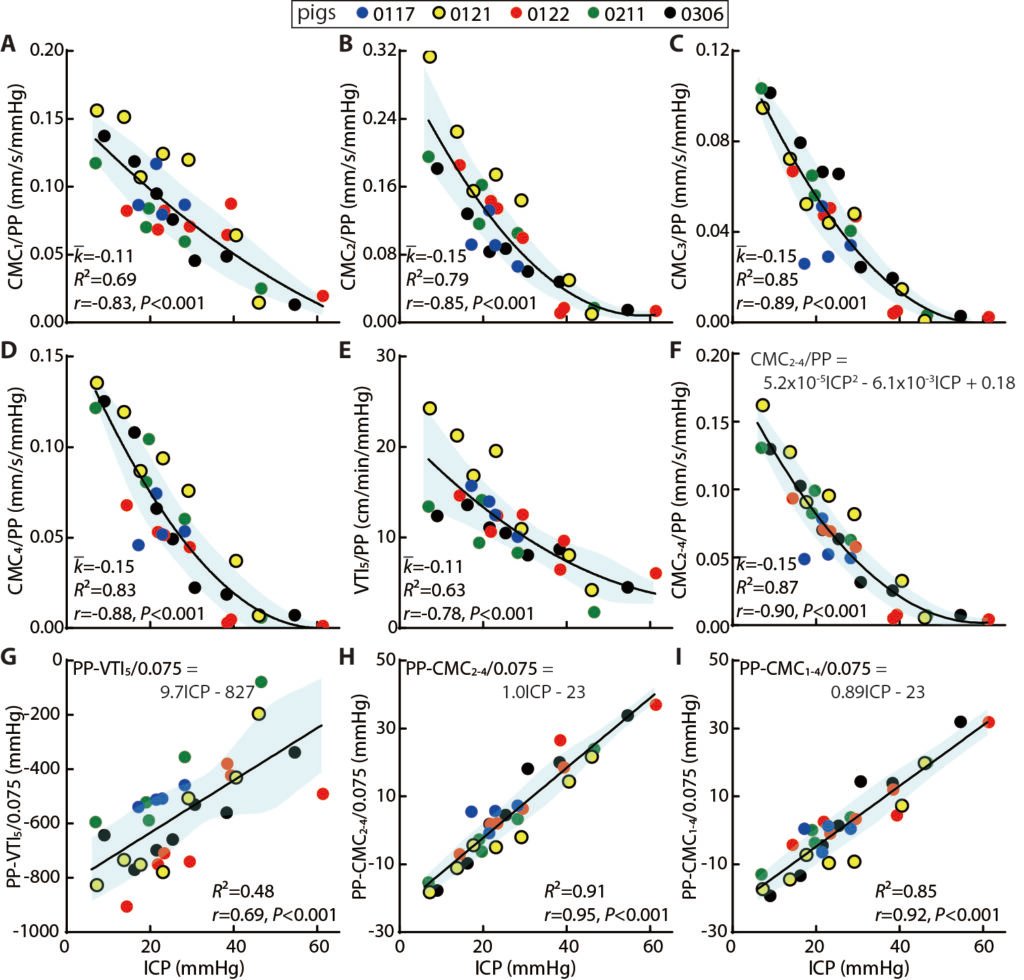
In order to obtain a reliable biomarker for ICP levels based on these perfusion parameters, we have investigated the relationships among the perfusion parameters, ICP, and other hemodynamic variables. Two relationships are discovered. As shown in Fig. 3 A-F scaling the perfusion parameters with PP yields nicely correlated trends for all regions which can be described by quadratic functions. Particularly, owing to its best fitting (R2=0.87) and highest correlation (r=-0.90, with P<0.001), the scaled collective cortical micro-perfusion (CMC2-4/PP) appears to be particularly suitable as an ICP biomarker. Fig. 3 G-I shows a convenient alternative linear trend to predict ICP, with r=0.92 and R2=0.85 for CMC1-4, and even higher values, r=0.95 and R2=0.91 for CMC2-4.